Mozambique Number of confirmed mpox cases rises to 13
Mozambique has none of the four India-made syrups flagged by WHO – AFARMO | Domingo report
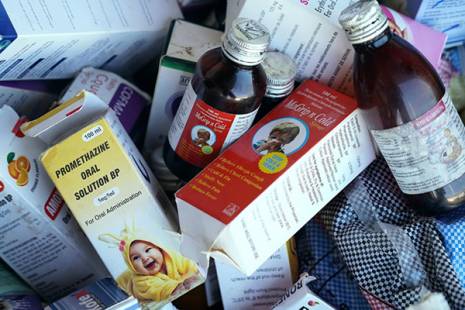
The president of the Association of Pharmacists of Mozambique (AFARMO), Lucien Pierre Nkunda Lukusa, told newspaper Domingo that Mozambique has none of the four cough syrups produced by an Indian company and linked to the acute renal failures which caused the death of around 70 children in the Gambia in the last three months.
The four paediatric cough syrups manufactured by Maiden Pharmaceuticals are called Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup.
World Health Organization (WHO) laboratory analysis has identified potentially toxic components in samples of the products.
READ: India tells Gambia it is probing childrens’ deaths linked to India-made drugs
“The marketing of medicines in Mozambique is well regulated, we have very strong legal mechanisms. The importer must be recognized, that is, carry an authorization for the purpose and the medicine registered in the country,” Nkunda Lukusa explained.
According to Lucien Pierre Nkunda Lukusa, Mozambique does not market any medicine from Maiden Pharmaceuticals.
He added that any medicine which leaves India for Mozambique must have a quality certificate, after undergoing a pre-shipment test.
Lucien Pierre Nkunda Lukusa said that the Mozambican authorities are on alert because there may be people who have recently travelled and may have acquired the syrups in other countries.
READ: Angolan government bans import and sale of syrups made in India
Nigeria warns against syrups linked to Gambia deaths
Moçambique não usa xaropes suspensos pela OMS
O presidente da Associação dos Farmacêuticos de Moçambique (AFARMO), Lukussa Pierre, garantiu ao jornal Domingo que no país não há xaropes para tosse e constipação produzidos por um laboratório indiano, associados a lesões renais agudas que causaram a morte de cerca de 70 crianças na Gâmbia nos últimos três meses.
Trata-se de four paediatric cough syrups namely Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup , do fabricante de medicamentos Maiden Pharmaceuticals.
Segundo a Organização Mundial da Saúde (OMS), análises laboratoriais identificaram componentes potencialmente tóxicos nas
amostras nos produtos em causa.
“A comercialização de medicamentos no país está bem regulamentada, temos mecanismos legais muito fortes. O importador deve ser reconhecido, ou seja, portar uma autorização para o efeito e o medicamento registado no país”, explicou o presidente da AFARMO.
De acordo com Pierre, Moçambique não tem nenhum medicamento da referida farmacêutica. Acrescentou que todo o medicamento que sai da Índia para Moçambique deve possuir um certificado de qualidade, denominado teste de pré-embarque.
Afirmou que as autoridades estão em alerta porque podem existir pessoas que viajaram recentemente e podem ter adquirido os fármacos noutros países.











Leave a Reply
Be the First to Comment!
You must be logged in to post a comment.
You must be logged in to post a comment.