Tributes paid after Nigeria keeper Rufai dies aged 61
Nigeria warns against syrups linked to Gambia deaths
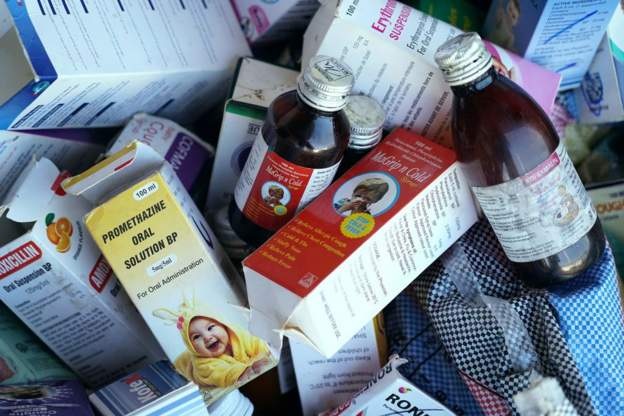
The Gambia has already started withdrawing the children medicines from circulation [Photo: AFP]
Nigeria has warned its citizens to be wary of the cough syrups linked to dozens of deaths of children in The Gambia.
The four syrups manufactured by Indian pharmaceutical company Maiden Pharmaceuticals are suspected to have been contaminated. The World Health Organization had issued a global alert over the paediatric medicines.
READ: WHO issues warning on Indian cough syrup linked to 66 Gambian child deaths
Nigeria’s National Agency for Food and Drug Administration and Control (Nafdac) has now asked people in Africa’s most populous country to avoid the said medicines.
In a statement, the agency says anyone who has used the “substandard products” or has suffered any adverse reaction, should seek immediate medical advice and report to the health authorities.
It says effects of the toxic substances allegedly contained in the cough syrups include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache and acute kidney injury which may lead to death.
But it has not said whether the syrups have been discovered to be in circulation in the country.
The products are Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup. Maiden Pharmaceuticals had failed to provide guarantees about their safety, the WHO said.
In a comment to the ANI news agency Maiden said it was shocked and saddened over the incident. The company said it followed Indian health protocols and was co-operating with an investigation.
The Gambia has already started withdrawing the medicines from circulation. At least 69 deaths of children have been linked to the cough syrups there.
The WHO had expressed fears that the Gambia might not be the only country where the cough syrups had been exported to.
Gambia cough syrup scandal: Police investigate deaths linked to Indian medicine
NAFDAC is notifying Healthcare providers and the public of four substandard products identified in The Gambia and reported to WHO in September 2022.
The four products are Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup.
— NAFDAC NIGERIA (@NafdacAgency) October 10, 2022
Toxic effects can include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state, and acute kidney injury which may lead to death.
According to WHO, the four cold and cough syrups in question have been potentially linked with acute kidney…
— NAFDAC NIGERIA (@NafdacAgency) October 10, 2022
NAFDAC implores importers, distributors, retailers and consumers to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale and use of the substandard cough syrups.
— NAFDAC NIGERIA (@NafdacAgency) October 10, 2022
If you have these substandard products, please DO NOT use them. If you, or someone you know have used these products or suffered any adverse reaction/event after use, you are advised to seek immediate medical advice from a qualified healthcare professional.
— NAFDAC NIGERIA (@NafdacAgency) October 10, 2022













Leave a Reply
Be the First to Comment!
You must be logged in to post a comment.
You must be logged in to post a comment.